
Top Performing Drug – Ozempic (September Edition)
Shots:
-
PharmaShots’ next episode on the top-performing drug of the month, based on 2021 revenue, focuses on Ozempic, a drug developed and marketed by Novo Nordisk
-
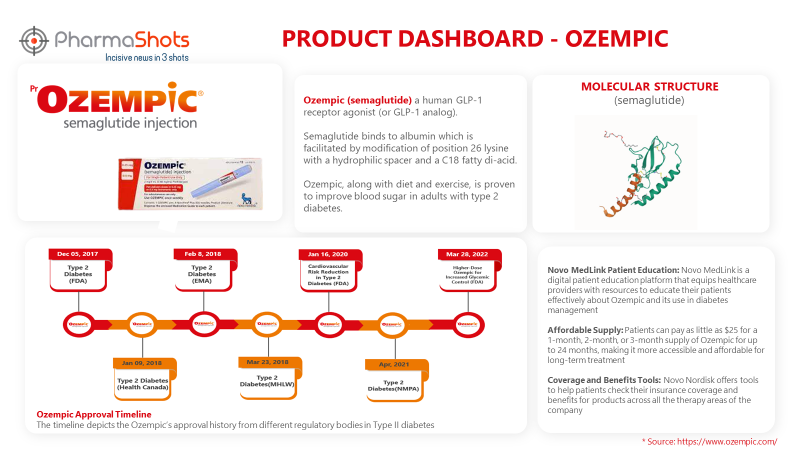
Ozempic (semaglutide) is a human GLP-1 receptor agonist (or GLP-1 analog). It, when taken along with diet and exercise, is proven to improve blood sugar in adults with type 2 diabetes
-
PharmaShots presents a concise take on the key features of Ozempic with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis, emotive patients' stories, and informative KOL reviews
Active Ingredient: semaglutide
Dosage Forms & Strengths:
Injection: 2 mg/3 mL (0.68 mg/mL and 1.34 mg/mL) available in single-patient-use pen that delivers 0.25 mg or 0.5 mg per injection
Injection: 4 mg/3 mL (1.34 mg/mL) available in single-patient-use pen that delivers 1 mg per injection
Injection: 8 mg/3 mL (2.68 mg/mL) available in single-patient-use pen that delivers 2 mg per injection
Mechanism of Action: GLP-1 receptor agonist
Originator: Novo Nordisk
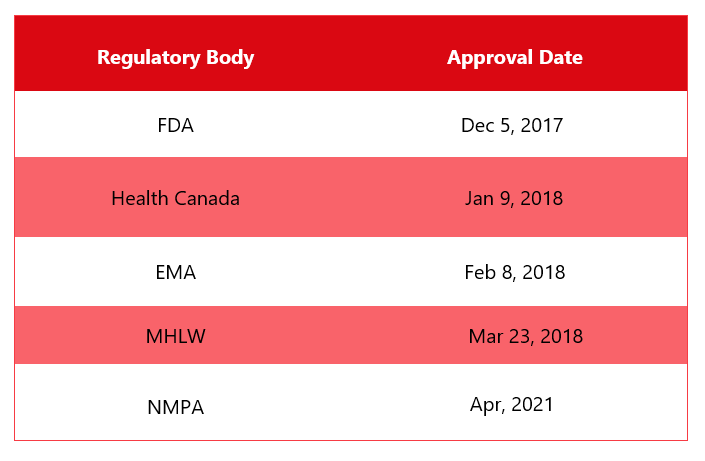
First Approvals: The table below depicts the first approvals of Ozempic from different regulatory agencies.
Revenue Analysis1
Developed and marketed by Novo Nordisk, Ozempic has been launched in around 75 countries. Driven by North American and international operations, Ozempic sales grew by 66% in 2022 vs. 2021. The graph below depicts the analysis of Ozempic’s past 5 years’ revenue.
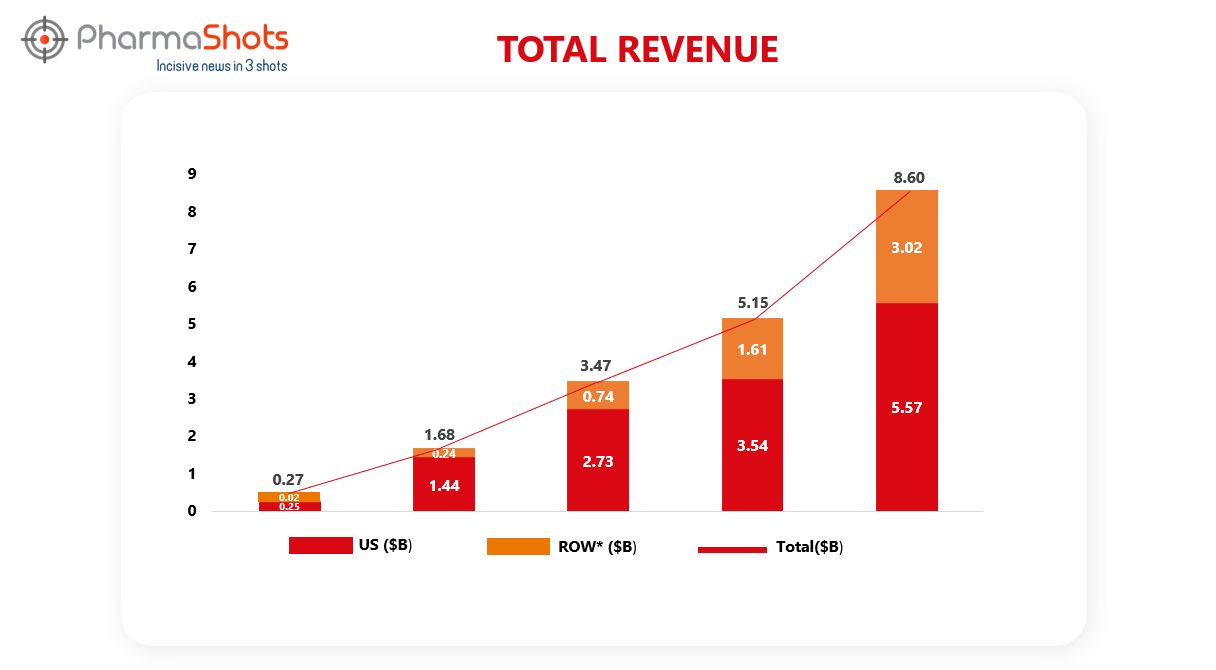
*ROW sales include sales from EMEA (Europe and the Middle East and Africa) China, Canada and other countries
Ozempic Approved Indications2
Ozempic is a GLP-1 receptor agonist indicated:
-
as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus
-
to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease
Clinical Trials Analysis3
Ozempic underwent 51 trials, incl. 19 industry trials, 15 of which were interventional and 4 observational. (Trials were taken on 11th Sep 2023).
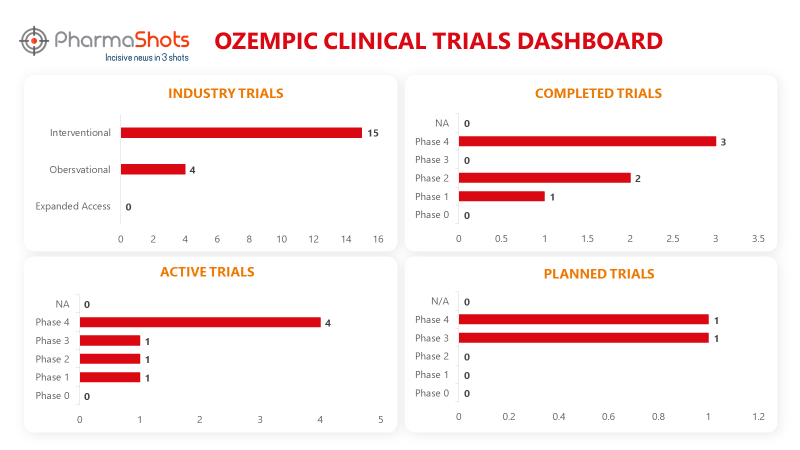
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Ozempic Trials Representation (Country-wise)4
The stacked column chart below represents the ongoing evaluation of Ozempic in various indications worldwide. It focuses on the top 10 countries globally and includes only interventional studies.
* The chart depicts data till 8th Sep 2023
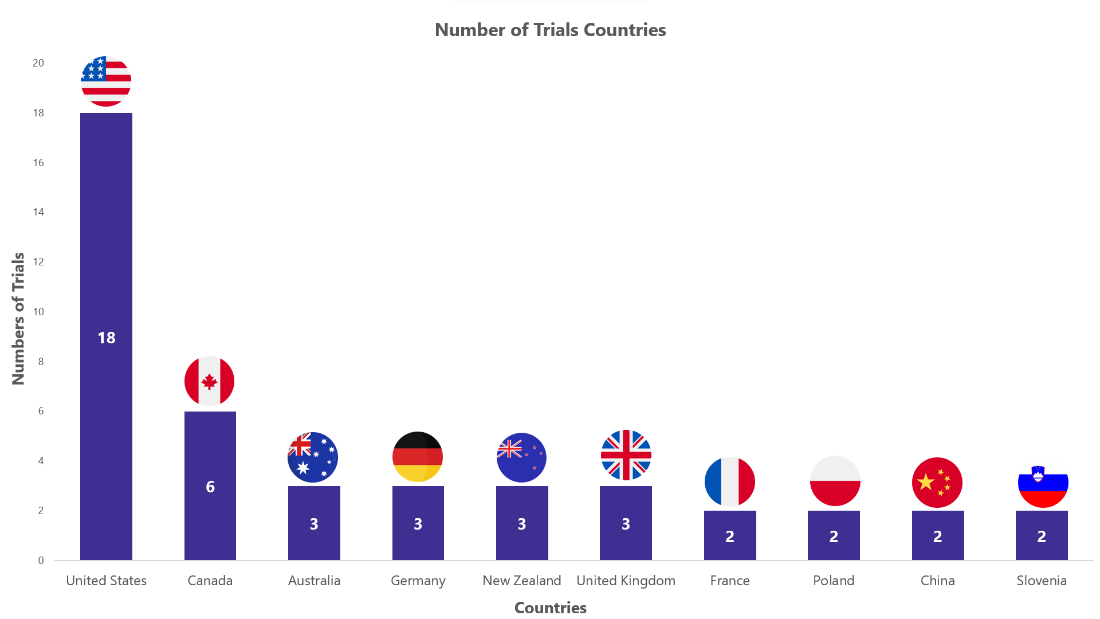
Besides the data shown in the chart, 17 countries have single-digit trial numbers. For a detailed report on it, mail us at connect@pharmashots.com
Product Dashboard
PharmaShots presents an illustrative infographic highlighting essential metrics and pertinent information about Ozempic.
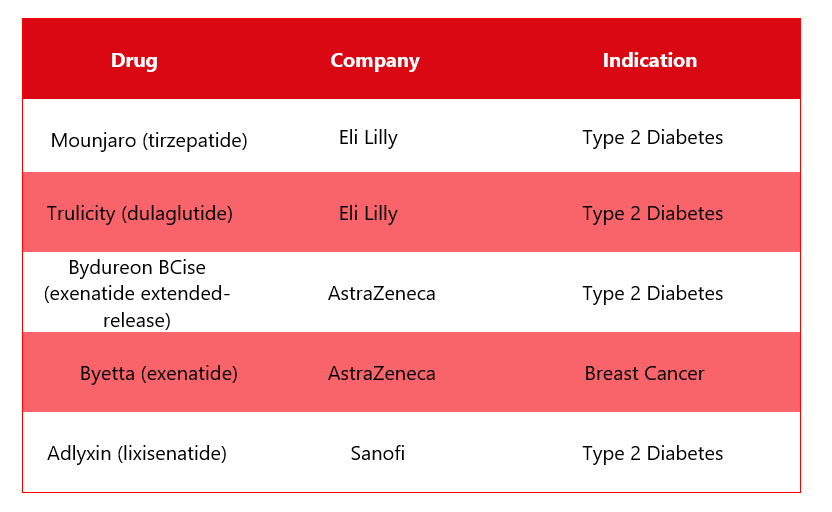
Ozempic’s Alternative Drugs5
In response to Ozempic, several other drugs are available in the market and are used to treat different indications. Some of the alternative drugs are shown in the table below:
SWOT Analysis6
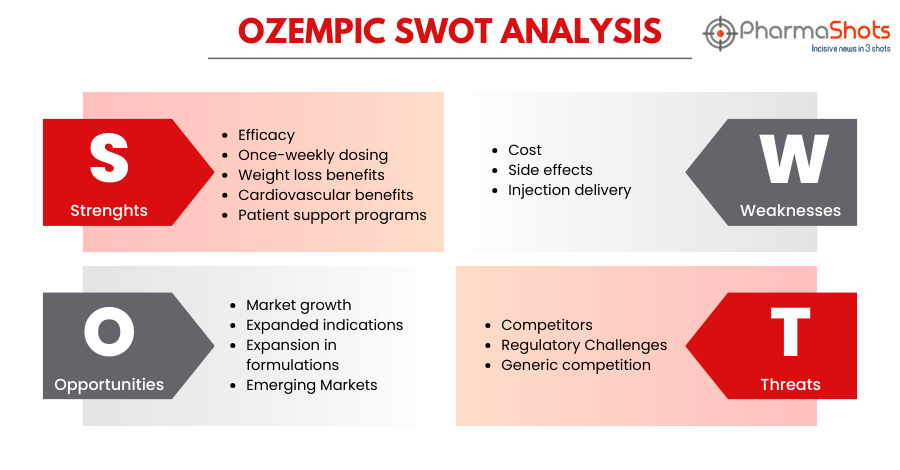
Strengths:
-
Efficacy: Ozempic, when taken along with diet and exercise, has demonstrated high effectiveness in lowering blood sugar levels in patients with type 2 diabetes, helping them achieve better glycemic control
-
Once-a-week dose: The treatment with Ozempic is a convenient treatment option as it is a once-a-week injection that reduces the burden of daily medication management for patients
-
Weight loss benefits: Although Ozempic is not a weight loss drug, several patients have experienced weight loss while taking Ozempic, which can be a significant advantage for those with type 2 diabetes, as obesity is a common comorbidity
-
Cardiovascular benefits: Ozempic lowers the risk of major CV events such as stroke, heart attack, or death in adults with known heart disease.
-
Patient support programs: Novo Nordisk, the manufacturer of Ozempic, offers patient support programs, including educational resources such as Novo MedLink Patient Education, which is a digital patient education platform that equips healthcare providers with resources to educate their patients effectively about their products, including Ozempic and its use in diabetes management
Weaknesses:
-
Cost: Ozempic can be expensive, making it a less affordable option for some patients, particularly those without insurance coverage.
-
Side effects: Like any medication, Ozempic may cause side effects, including nausea, vomiting, diarrhea, stomach pain, etc.
-
Injection delivery: Self-administering injections may cause discomfort and inconvenience to some patients
Opportunities:
-
Market growth: With the rising prevalence of type 2 diabetes globally, there is a growing market for diabetes medications like Ozempic.
-
Expanded indications: Ozempic could potentially expand its use beyond type 2 diabetes treatment, such as in weight management or prediabetes prevention.
-
Expansion in formulations: Continued research and development efforts may lead to its expansion in different formulations that can provide ease for the patients in the administration of the drug
-
Emerging Markets: Ozempic has been launched in 75 countries already. The growing demand for effective drugs for type 2 diabetes can bring an opportunity for Ozempic to expand its market presence globally
Threats:
-
Competitors: The market for diabetes medications is highly competitive, with numerous alternative treatments available, including oral medications, other injectables, and emerging therapies
-
Regulatory Challenges: Changes in healthcare regulations and reimbursement policies could affect the availability and affordability of Ozempic
-
Generic competition: Ozempic may face competition from generic versions, potentially reducing its market share and profitability after the expiration of its patent
Patient Stories7
Patients' stories have been instrumental in sharing their perspectives on individual experiences in facing health challenges. They serve as essential resources for gaining a deeper understanding of healthcare services and policies. Below, you will find selected patient testimonials related to Ozempic.
-
Maria‘s Story: Maria belongs to a Hispanic family with a history of having type 2 diabetes, Maria felt empowered to share her story. Now, with diet, exercise, and taking Ozempic as prescribed by her doctor Maria has lowered her A1C by under 7% and has lost some weight. She said,” Now I see the positive impact that choosing to prioritize my type 2 diabetes management has on my family, and that motivates me.”
-
Jane’s Story: Jane manages her type 2 diabetes through exercise and healthy eating habits along with a weekly dose of Ozempic. She has lowered her A1C by under 7% and has lost some weight. She said, “By letting people know about my story, I hope I can be motivational and help them on their own path of managing type 2 diabetes."
-
Susan’s Story: Susan was diagnosed with prediabetes, meaning her blood sugar levels were higher than normal, but not high enough to be considered type 2 diabetes. Her doctor prescribed Ozempic off-label. Susan was aware of Ozempic because her 54-year-old husband, Michael, began taking it about six months earlier to treat his type 2 diabetes and bring down his A1C to under 6. She said, “Seeing Michael’s success absolutely influenced me wanting to try it”
KOL* Reviews8
KOL reviews are valuable resources for any drug to increase its reach and reliability. Generally, these reviews are helpful when consumers research the product and read multiple reviews before buying it. Below are some of the KOL reviews for Ozempic.
In June 2023, Novo Nordisk unveiled a series of measures to safeguard US patients from the illegal promotion and distribution of counterfeit and compounded semaglutide products that falsely claim to contain semaglutide, while also emphasizing the responsible usage of Novo Nordisk's FDA-approved medications.
1. Dr Juan Pablo Frias, Medical Director of Velocity Clinical Research said:
"Type 2 diabetes is a complex disease that can progress over time even if a person is managing it with medication, diet and exercise. With its proven safety and efficacy, Ozempic helps deliver on blood glucose control and offers major cardiovascular event risk reduction in adults with type 2 diabetes and known heart disease, plus it can help many patients lose some weight. With a 2 mg dose, we have an additional option so patients can stay on the same medication therapy even if their blood sugar needs shift."
2. Todd Hobbs, Novo VP and U.S. CMO said:
"Today's milestone establishes Ozempic as an option for patients to help address two critical aspects of managing type 2 diabetes, blood sugar control and cardiovascular risk reduction, in those with known heart disease.”
Ozempic was approved in 2017 by the US FDA as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus.
3. Doug Langa, EVP, North America operations and President of Novo Nordisk said:
"Our priority is to ensure that patients have a safe and positive experience with our FDA-approved semaglutide medicines, and these actions are a direct reflection of that focus. We believe it's important to provide additional tools and education to support the proper use of our approved semaglutide products and create broad public awareness regarding the difference between our FDA-approved medicines and other products being labeled as semaglutide."
After Ozempic’s approval in 2017, it quickly ascended to blockbuster status after its launch in early 2018. Within the first nine months of 2019, Ozempic achieved global sales of $1 billion.
4. Mads Krogsgaard Thomsen, Novo EVP and CSO said:
"We are very excited about the first approval of Ozempic® and look forward to making this important innovation available to people in the US with type 2 diabetes in the beginning of 2018. Type 2 diabetes is a complex disease, but with the unique clinical profile of Ozempic®, we believe it has the potential to set a new standard for the treatment of the disease."
In March 2022, Ozempic 2 mg dose was approved by the US FDA to improve blood sugar in adults with type 2 diabetes and to reduce the risk of major cardiovascular events such as heart attack, stroke, or death in adults with type 2 diabetes and known heart disease.
5. Martin Lange, EVP, Development at Novo Nordisk said:
“We are pleased with the FDA approval for a higher 2.0 mg dose of Ozempic®, which further supports our purpose of driving change in diabetes care. The approval of the 2.0 mg dose allows more people with type 2 diabetes to achieve and maintain individualized glycaemic targets and remain on the same medication for longer as their needs evolve.”
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
-
Ozempic’s Alternatives
6. SWOT Analysis
7. Patient stories
8. KOL Reviews
Related Post: Top Performing Drug – Ibrance (August Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














